3.10 - Atmospheric Moisture
Watch: Instructor's Lecture Links to an external site.
Revisiting Evaporation and Condensation
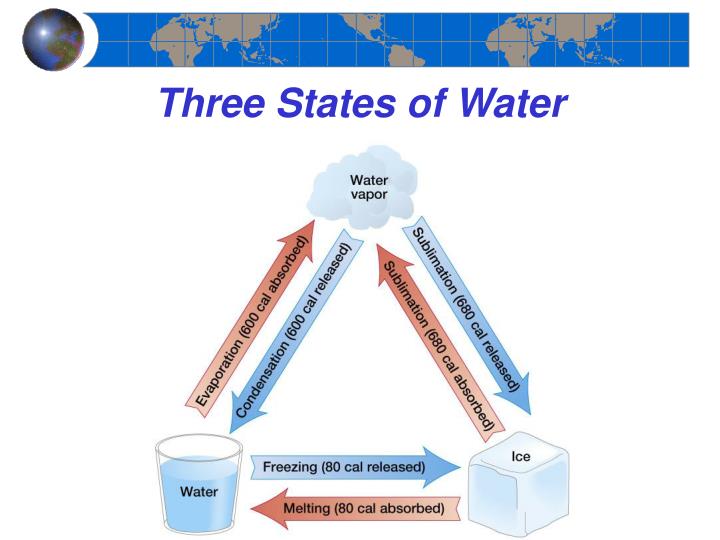
As we have discussed, when water changes state from a liquid to a gas that is evaporation. Evaporation is a cooling process. Latent heat is stored by the water vapor molecules and is released during condensation. Because latent heat is released, condensation is a warming process. We say that the air is saturated if the addition of any more water vapor to a mass of air leads to condensation. A poor analogy is a sponge. Once that sponge is at its capacity, holding as much water as it can, it will not be able to "hold" anymore and we say that sponge is saturated.
A number of factors impact the rate at which water evaporates. Wind speed affects the rate at which the water evaporates. As the wind blows, it sweeps away water vapor molecules that are in the air. The amount of water vapor in the air in the region of this evaporation is reduced, which allows more water molecules to evaporate. Wind can also change the vapor pressure by moving air about rapidly, thereby causing it to expand. This process creates room for extra water vapor and evaporation will continue to occur while the wind is blowing.
The temperature of the air and water also impact evaporation. In warmer water, more water molecules have enough energy to break free of the liquid water and enter the atmosphere. In warm air, water vapor molecules are moving fast enough that they are unlikely to reform hydrogen bonds with other water vapor molecules and are likely, therefore, to remain in their gaseous state. They have too much energy and motion to reform hydrogen bonds and condense.
Humidity
Humidity refers to the water vapor in the atmosphere. We measure humidity in a number of ways. Absolute humidity, specific humidity and relative humidity are three common ways we describe humidity. In weather, the most common measure is the relative humidity. Relative humidity is the amount of water vapor in the air compared to the total amount needed for saturation. If the relative humidity is 100%, the air is saturated . Relative humidity can be expressed as a formula:
RH = AH/P x 100
AH = the amount of water vapor present
P = the water vapor capacity of the air at a given temperature
There are two ways to change relative humidity: change the amount of water vapor in the air or change the water vapor capacity of the air. If evaporation increases, the amount of water vapor increases and the relative humidity will rise. If condensation increases, the amount of water vapor present decreases and the relative humidity will drop.
The most common and important change in relative humidity comes from changing the capacity of the air to hold water vapor. This is accomplished by heating or cooling the air. If we cool the air, its ability to hold water vapor drops and therefore, its relative humidity goes up. If we heat the air, its ability to hold moisture increases and the relative humidity drops. Saying that the air "holds moisture" is a simplification that isn't the best way to explain why as the temperature changes, the relative humidity changes inversely. Check out my video and this article Links to an external site. to understand what is actually happening as we change the temperature of air.
We see this process at work over the course of a day. At the hottest point in the afternoon, between 3 p.m. and 6 p.m, the relative humidity is lowest (if nothing interesting is happening with the weather). Plants will wilt in the afternoon heat because of this drop in relative humidity. The relative humidity is likely to be the highest at the coldest point in the day, usually shortly before dawn. In the diagram below, the relative humidity is changing over the course of a day, not because the amount of water vapor in the air has changed but because the air's capacity has changed due to changes in temperature.

Dew Point Temperature
The critical temperature at which saturation is reached and condensation begins is the dew point temperature. It varies with the moisture content of the air. Dew point temperature is based on how much water vapor is in the air. So while dew point is expressed in terms of temperature, it is really a measure of humidity. Most people find a dew point temperature of less than 60°F to be comfortable. When the dew point rises to 65°F and above, more and more people find it uncomfortable. The higher the dew point temperature the more moisture in the air.
When water vapor condenses and releases latent heat, the air is warmed a little. This means that overnight as the air cools to dew point condensation will begin slowing the temperature fall. If no fronts move in or other major atmospheric changes occur, the air isn't likely to get much colder than dew point temperature overnight.
If the air that reaches its dew point is on the ground or near the ground; dew, frost or fog forms. Air near the ground is most often cooled because it is in contact with the ground and as the ground cools through radiation the air cools with it. Air rising in the atmosphere, may eventually cool to dew point temperature. If it does water will condense around nuclei and droplets will grow as they bump into one another and we may have haze, clouds and rain.
How is Relative Humidity Measured
 Relative humidity can be measured by an instrument called a hygrometer. The simplest hygrometer - a sling psychrometer - consists of two thermometers mounted together with a handle attached on a chain. One thermometer is ordinary. The other has a cloth wick over its bulb and is called a wet-bulb thermometer. When a reading is to be taken, the wick is dipped in water and then the instrument is whirled around. During the whirling, the water evaporates from the wick, cooling the wet-bulb thermometer. Then the temperatures of both thermometers are read. If the surrounding air is dry, more moisture evaporates from the wick, cooling the wet-bulb thermometer more, causing there to be a greater difference between the temperatures of the two thermometers. If the surrounding air is holding as much moisture as possible - if the relative humidity is 100% - there will be no difference between the temperatures on the two thermometers. Meteorologists have worked out charts of these differences for each degree of temperature so that the observer can find relative humidity easily.
Relative humidity can be measured by an instrument called a hygrometer. The simplest hygrometer - a sling psychrometer - consists of two thermometers mounted together with a handle attached on a chain. One thermometer is ordinary. The other has a cloth wick over its bulb and is called a wet-bulb thermometer. When a reading is to be taken, the wick is dipped in water and then the instrument is whirled around. During the whirling, the water evaporates from the wick, cooling the wet-bulb thermometer. Then the temperatures of both thermometers are read. If the surrounding air is dry, more moisture evaporates from the wick, cooling the wet-bulb thermometer more, causing there to be a greater difference between the temperatures of the two thermometers. If the surrounding air is holding as much moisture as possible - if the relative humidity is 100% - there will be no difference between the temperatures on the two thermometers. Meteorologists have worked out charts of these differences for each degree of temperature so that the observer can find relative humidity easily.
