3.9 - Properties of Water
The Water Molecule
 We live on a planet dominated by water. 70% of the Earth's surface is covered by water. Water is, of course, essential for life and is the major constituent of almost all life forms on Earth.
We live on a planet dominated by water. 70% of the Earth's surface is covered by water. Water is, of course, essential for life and is the major constituent of almost all life forms on Earth.
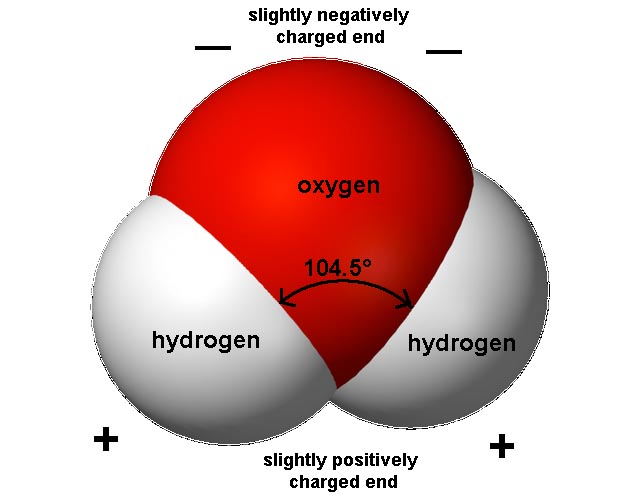
Water has a very simple atomic structure. The structure consists of two hydrogen atoms bonded to one oxygen atom. The nature of the atomic structure of water causes its molecules to have unique properties. The hydrogen side of the water molecule has a slight positive charge. On the other side of the molecule a negative charge exists. This molecular polarity gives water many of its unique characteristics.
The Properties of Water
Watch: Instructor's Lecture
Links to an external site.
 Water molecules are the only substance on Earth that exist in all three physical states of matter: solid, liquid, and gas at common temperatures. Whenever water changes state (liquid to gas etc) there are massive amounts of heat exchanged. This feature plays an important role in the redistribution of heat energy in the Earth's atmosphere. In terms of heat being transferred into the atmosphere, approximately 3/4's of this process is accomplished by the evaporation and condensation of water.
Water molecules are the only substance on Earth that exist in all three physical states of matter: solid, liquid, and gas at common temperatures. Whenever water changes state (liquid to gas etc) there are massive amounts of heat exchanged. This feature plays an important role in the redistribution of heat energy in the Earth's atmosphere. In terms of heat being transferred into the atmosphere, approximately 3/4's of this process is accomplished by the evaporation and condensation of water. When water freezes it expands. The freezing of water molecules causes their mass to occupy a larger volume. When water freezes it expands rapidly adding about 9% by volume. This is why ice cubes float and so does the ice on a frozen lake
When water freezes it expands. The freezing of water molecules causes their mass to occupy a larger volume. When water freezes it expands rapidly adding about 9% by volume. This is why ice cubes float and so does the ice on a frozen lake- Water has a high specific heat. Links to an external site.Specific heat is the amount of energy required to change the temperature of a substance. Because water has a high specific heat, it can absorb large amounts of heat energy before it begins to get hot. It also means that water releases heat energy slowly when it cools.
- Water in a pure state has a neutral pH Links to an external site.. As a result, pure water is neither acidic Links to an external site. nor basic Links to an external site..
- Water is a universal solvent. It is able to dissolve a large number of different chemical compounds. This feature also enables water to carry dissolved nutrients in runoff Links to an external site., infiltration Links to an external site., groundwater flow Links to an external site., and living organisms.
:max_bytes(150000):strip_icc()/139802493-56a12f615f9b58b7d0bcde91.jpg) Water has a high surface tension.
Links to an external site.Water molecules adhere to one another (ie. they stick to each other) so they form drops rather than spread out in a thin film
Links to an external site.. Water molecules also cohere to other polar molecules (ie water sticks to paper towels and soil etc.) Water's high surface tension allows for the formation of water droplets and waves, allows plants to move water (and dissolved nutrients) from their roots to their leaves, and the movement of blood through tiny vessels in the bodies of some animals.
Water has a high surface tension.
Links to an external site.Water molecules adhere to one another (ie. they stick to each other) so they form drops rather than spread out in a thin film
Links to an external site.. Water molecules also cohere to other polar molecules (ie water sticks to paper towels and soil etc.) Water's high surface tension allows for the formation of water droplets and waves, allows plants to move water (and dissolved nutrients) from their roots to their leaves, and the movement of blood through tiny vessels in the bodies of some animals.
Watch this fun video that demonstrates water's high surface tension.
Links to an external site.
Phase Changes of Water
Watch: Instructor's Lecture Links to an external site.
The great majority of the world's moisture is in the form of liquid water, which can be converted to the gaseous form (water vapor) by evaporation or to the sold form (ice) by freezing. Water vapor can be converted to liquid water by condensation or directly to ice by sublimation. In each of these phase changes there is an exchange of latent heat energy. This energy is the engine of weather on our planet.
Evaporation
Molecules are always moving. A substance's temperature depends on how fast molecules are moving - the faster the molecule moves the hotter the substance. Temperature is one of the factors which determine if a substance is a solid, liquid or gas.
Let's imagine we have superhuman vision. We are watching individual water molecules zip around in a glass of water. Imagine you are watching a particularly speedy molecule. If its moving fast enough and in the right direction, you might see it break the attractive force of water's high surface tension (hydrogen bonds) and enter the atmosphere as water vapor. If we warm up the water, more molecules will be moving fast enough to break the water's surface tension and enter the atmosphere as water vapor. This requires a great deal of energy (540 calories per gram) . These molecules "take" this energy from the body of water which they are leaving (in this case, our glass of water) and store it. This stored energy is the latent heat of vaporization. Therefore, evaporation is a cooling process.
Condensation
In order for water molecules that have changed phase from liquid to gas to return to the liquid state, you need to slow those water vapor molecules down. In the atmosphere, water vapor molecules are zipping around bumping into each other, and bouncing off one another. In order for them to reform a hydrogen bond with other water vapor molecules and return to the liquid state, you have to slow them down. This is accomplished by dropping the temperature of the air. But water vapor molecules need more help reforming hydrogen bonds and condensing. They need another molecule to stick to. These are called condensation nuclei. Smoke, ash, dirt particles, sea salt - any solid particle in the atmosphere, particularly if it is another polar molecule will do. Cool the air off and give those water vapor molecules something to stick to and condensation will occur. The latent heat stored during evaporation is released to the surrounding atmosphere during condensation. Condensation, therefore, is a warming process.