3.3 - The Sun's Energy: The Electromagnetic Spectrum
The Angle of the Sun and the Atmosphere
Watch: Instructor's Video Links to an external site.
 The atmosphere has a strong influence over the amount of solar insolation reaching the surface of the Earth. The Earth's atmosphere absorbs, reflects, scatters and transmits incoming radiation. The "longer the trip" through the Atmosphere the less solar radiation will "make it" to the surface of the Earth. It is more likely to be scattered or reflected. A 90° angle creates the shortest "trip" through our atmosphere for incoming radiation (b. on the diagram). The more oblique the angle the longer the trip and the more energy which is lost (a. on the diagram).
The atmosphere has a strong influence over the amount of solar insolation reaching the surface of the Earth. The Earth's atmosphere absorbs, reflects, scatters and transmits incoming radiation. The "longer the trip" through the Atmosphere the less solar radiation will "make it" to the surface of the Earth. It is more likely to be scattered or reflected. A 90° angle creates the shortest "trip" through our atmosphere for incoming radiation (b. on the diagram). The more oblique the angle the longer the trip and the more energy which is lost (a. on the diagram).
The energy received at the surface of the Earth is further impacted by the nature of the surface it strikes. A certain amount may be absorbed or reflected back into the atmosphere. The amount reflected is called a surface's albedo. In addition, the Earth emits radiation. Incoming and outgoing radiation maintain a balance over the globe as a whole and keep the overall temperature of the Earth fairly balanced.

The Sun's Energy

The Sun is the major source of energy for the Earth and the atmosphere. A little energy is released from within the Earth from the decay of radioactive materials, but the major energy source for life on earth is clearly the Sun. The energy from the Sun comes to us in the form of electromagnetic waves. While we are most familiar with visible light, the Sun is also sending energy in the form of x-rays, ultraviolet rays, infrared rays and even microwaves and radio waves.

Electromagnetic waves consist of magnetic fields and electric fields that unlike waves in water, do not need any medium to travel through - they can travel through a vacuum which is why they can travel through space. Electromagnetic radiation is a stream of photons traveling at 186,000 miles per second, the speed of light. It takes solar radiation 8 minutes to get here from the Sun. Only a fraction of the Sun's energy makes it through our atmosphere and strikes the Earth's surface.
The different electromagnetic waves emitted by the Sun are classified based on their wavelengths (the distance from one wave crest to the next) and in turn their frequency. All solar radiation travels at the same speed but has different wavelengths and frequencies. Shorter wavelengths mean higher frequency; while longer wavelengths mean lower frequency. Shortwave lengths mean that the waves are closer together and so more wavelengths transit in a given period of time than if the wavelengths were longer. We can, therefore, say that they have a higher frequency. On the diagram above, you can see that the very shortest wavelengths emitted by the Sun are gamma rays and x-rays. At the longer end of the spectrum are microwaves and radio waves. The hotter an object is the shorter the wavelengths it will emit on average. The Sun, therefore, emits primarily shortwave radiation as compared to the much cooler Earth which emits longer wave, infrared radiation.
The energy source for these waves is the Sun's interior. Very high temperatures and enormous pressure convert hydrogen to helium creating essentially nuclear fusion. The rate of internal production of energy output by the Sun is fairly constant. Energy output varies on an 11 year sunspot cycle with slightly increased production during periods of increased sunspot activity. This variation in solar energy has been seized upon by climate change deniers but the science Links to an external site. does not back up their conclusions.
We call the amount of energy produced by the Sun the solar constant (2 calories per square centimeter per minute). A calorie is the the amount of energy it takes to raise the temperature of one gram of water by 1 degree Celsius. Orbiting satellites have provided us with precise data on the solar constant.
Heat vs Temperature
Heat and temperature are obviously related but they aren't the same thing. Temperature is the measure of average speed of molecular movement within an object or substance. We can measure temperature with a thermometer. The higher the temperature the faster molecules move. Heat is the total kinetic energy of the atoms of a substance. Heat is the energy transferred between materials or systems due to their temperature differences. Heat travels from a hotter object to a colder object.
Most of us have observed that if you place a hot object (say a cup of tea) or a cold object (say a glass of ice water) in a room at ordinary temperatures, the object will tend toward thermal equilibrium with its environment. That is, the hot cup of tea gets colder and the ice water get warmer; the temperature of each approaches the temperature of the room.
How does heat move?
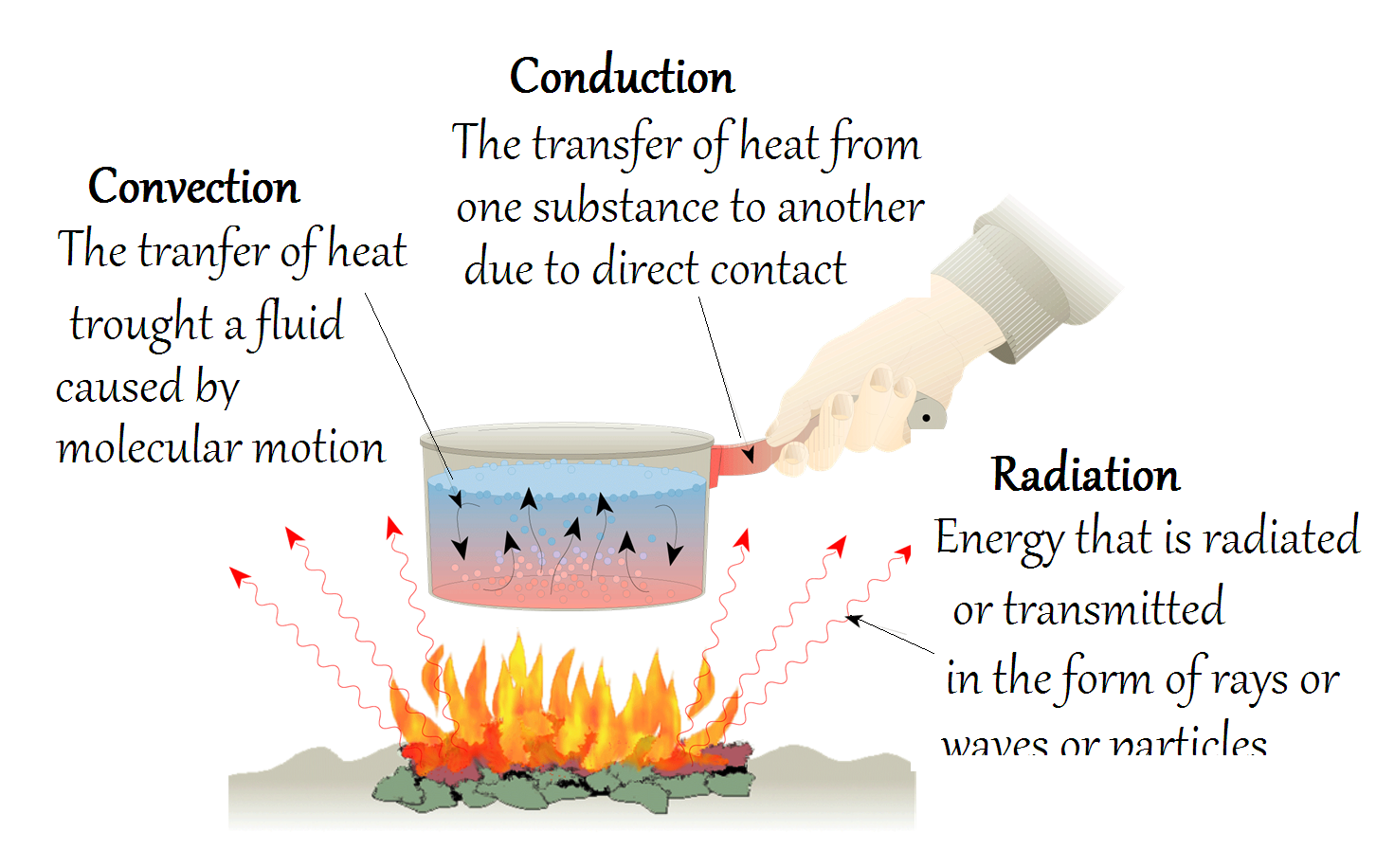 Heat is transferred through Earth's systems in three ways: conduction, convection, and radiation. Let's use this pot of water mysteriously floating over this fire as an example of each.
Heat is transferred through Earth's systems in three ways: conduction, convection, and radiation. Let's use this pot of water mysteriously floating over this fire as an example of each.
Why does the pot's handle get hot? The handle is hot because metal is a good conductor. Conduction is the transfer of heat by the collisions of molecules. The fire causes the molecules in the pan's bottom to vibrate faster. These vibrating molecules collide with their neighbors making them vibrate. After a while, molecules in the pan and handle are vibrating so fast that it is too hot to touch. Conduction occurs in solids, liquids and gases. Some objects (like the pan) are good conductors. Copper is an example of an excellent conductor. Others are insulators. Both soil and air are poor conductors. They are, however, good insulators.
What about the water in the pot? How is that being heated? Convection is the transfer of heat by the movement of a heated fluid (or gas). Most fluids (or gases) expand when heated. They become less dense and rise as a result of the increased buoyancy. Circulation caused by this effect accounts for the uniform heating of the water in the pot (or the air in a heated room). By definition, convection can only occur in a liquid or a gas and is a very efficient means of heat transfer in both.
And lastly, we have the nice warming effect of the flames as we stand near the fire. How is the heat from the fire transferred to us? The energy from the fire is being transferred primarily by radiation. Radiation is the transfer of heat as waves. Of these three transfer mechanisms, it is the only one that can travel through the vacuum which is space plus the atmosphere and its gases, land surfaces and water surfaces. Liquids, solids, and gases all can radiate.