Pre Lab Quiz: Water I
- Due Jan 23 at 10:30am
- Points 7.5
- Questions 10
- Available Jan 10 at 10pm - Jan 28 at 11:59pm
- Time Limit None
- Allowed Attempts 5
Instructions
Before you come to lab, I'd like you to have read and understood the basic material about the properties of water. This quiz allows you to check your understanding. As usual, you have 3 attempts to take the quiz and it is open book, open notes, but do not do it with another person.
The information you need to answer these questions can be found in your lab textbook- Water Lab and in the material and video below.
Biologist spend a lot of time thinking about water since it is so vital for life. A large portion of our bodies and cells are made of water. You may think you are a terrestrial organism, but in reality we are a large bag of water with a waterproof layer (our skin) that keeps us from drying out and blowing away in the dry, dry world we inhabit. Every cell is bathed in a solution of water, and contains mostly water with molecules dissolved in the water and organelles suspended in the water. In this lab, we will examine some important ideas/hypotheses about water and see if we can make accurate predictions that are borne out by the evidence.
Introduction Properties of Water
Water is a simple molecule (two hydrogens and one oxygen), but the way the atoms are bound together creates unique and remarkable characteristics. Covalent chemical bonds are bonds in which electrons are shared between two atoms. The two lines that connect the two hydrogen atoms to the oxygen atom represent covalent bonds:
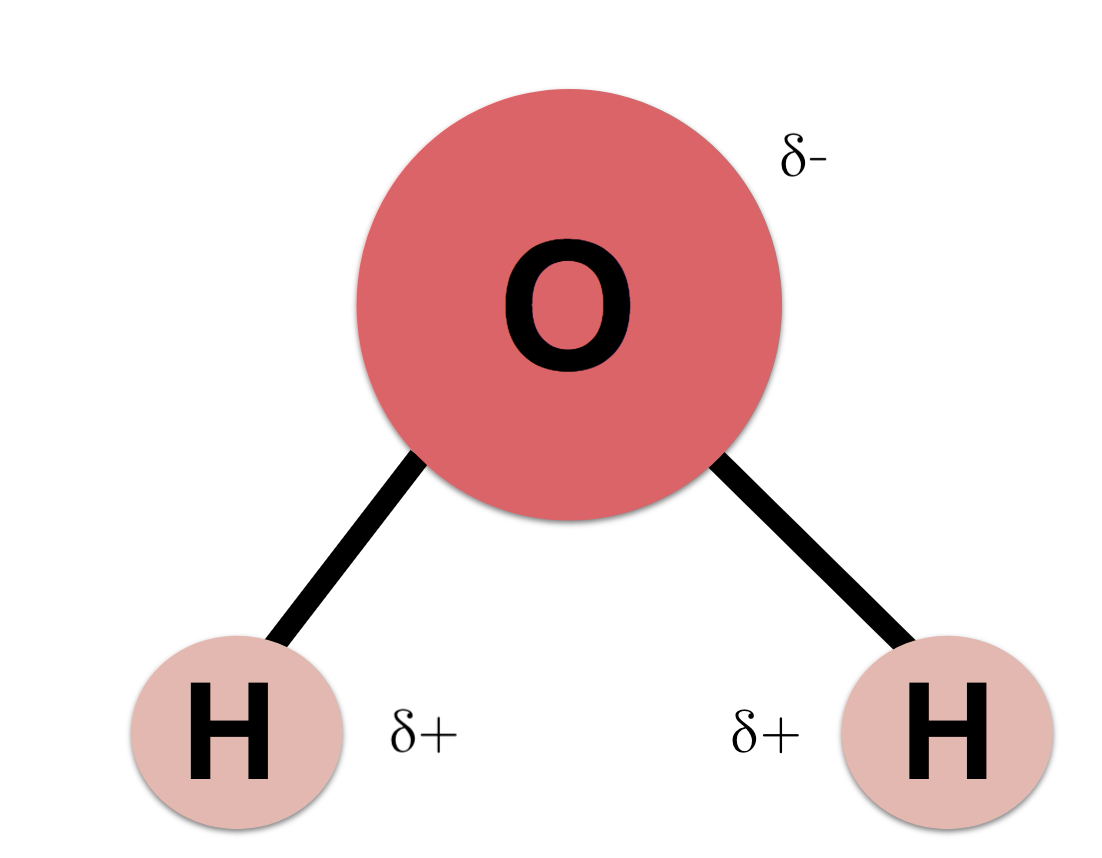
In water, the electrons are not shared equally. The oxygen “hogs” the electrons from the hydrogens, resulting in a partial charge on each atom: δ+ means partial positive charge; δ– means partial negative charge.
This makes the molecule like a small magnet. Any molecule that has a positive end and a negative end is called a polar molecule. Polar molecules are attracted to other polar or partially charged molecules. This attraction is called a hydrogen bond. Water is one of the most polar molecules.
Notes: Hydrogen bonds are not chemical bonds, merely attractions.
Text description of three connected water molecules graphic (Word doc).

The polarity and hydrogen bonds lead to many interesting qualities of water. In today’s lab, we will examine the properties of water; its polar nature, how it functions as a solvent,and its neutral pH, In addition, we will look at movement of water across semi–permeable membranes (osmosis).
Watch this video on Properties of Water
And then take this quiz